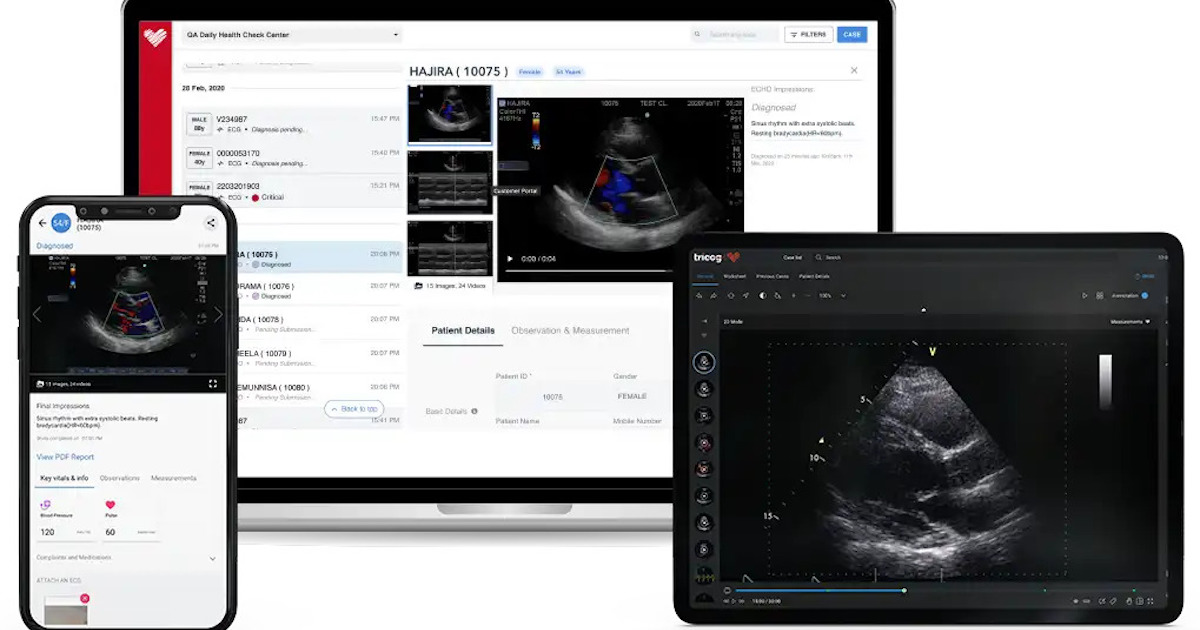
Tricog snaps up $9M funding for US expansion
AI-based cardiac diagnosis solution provider Tricog has raised Rs 700 million ($8.5 million) in a Series B2 funding round led by Japanese institutions, Omron and Sony Innovation Fund. The round was also participated by the University of Tokyo Edge Capital, Inventus Partners, and Singapore’s SG Innovate.
The startup plans to use its fresh funds for its global expansion, particularly its entry into the United States market.
Bangladeshi online pharmacy MedEasy acquires competitor
MedEasy, an online pharmacy in Bangladesh, has acquired fellow startup Alchemy for an undisclosed sum.
Through this acquisition, MedEasy is expanding its footprint by taking on Alchemy’s existing customer base. It will also be integrating the latter’s systems and processes into its own.
Lunit integrates pathology AI into Indica Labs’ platform
US-based digital pathology solution provider Indica Labs and South Korean medical AI company Lunit have entered into an integration partnership.
Based on a press statement, Lunit has integrated its AI pathology solutions, including the Lunit SCOPE PD-L1 for non-small cell lung cancer, into Indica Labs’ HALO AP clinical image management platform.
Their combined solution, which will enable pathologists and labs to “deliver faster, more accurate, and more reproducible answers,” is now being used at precision oncology company Guardant Health.
Coreline Soft enters Latin American market
Coreline Soft has received clearance for its line of AI-powered medical imaging solutions, AVIEW, from the Brazilian Health Regulatory Agency or Anvisa.
This marks the company’s first entry into the Latin American market, following its earlier entry into other major markets, including the United States, Europe, Japan, Taiwan, Singapore, and Australia.
Korean researchers develop wearable piezoelectric BP sensor
A research team from the Korea Advanced Institute of Science and Technology has developed a wearable piezoelectric blood pressure sensor, which is said to be more sensitive compared to its PPG-based counterpart.
According to a press release, ultrathin piezoelectric sensors exhibit “conformal contact” with the skin to collect accurate blood pressure from subtle pulses.
A clinical trial at St. Mary’s Hospital of the Catholic University of Korea has validated the accuracy of the piezoelectric sensor, recording errors within ±5 mmHg and a standard deviation under 8 mmHg for both systolic and diastolic blood pressure.
The reseachers have also embedded the sensor into a watch to enable continuous BP monitoring.