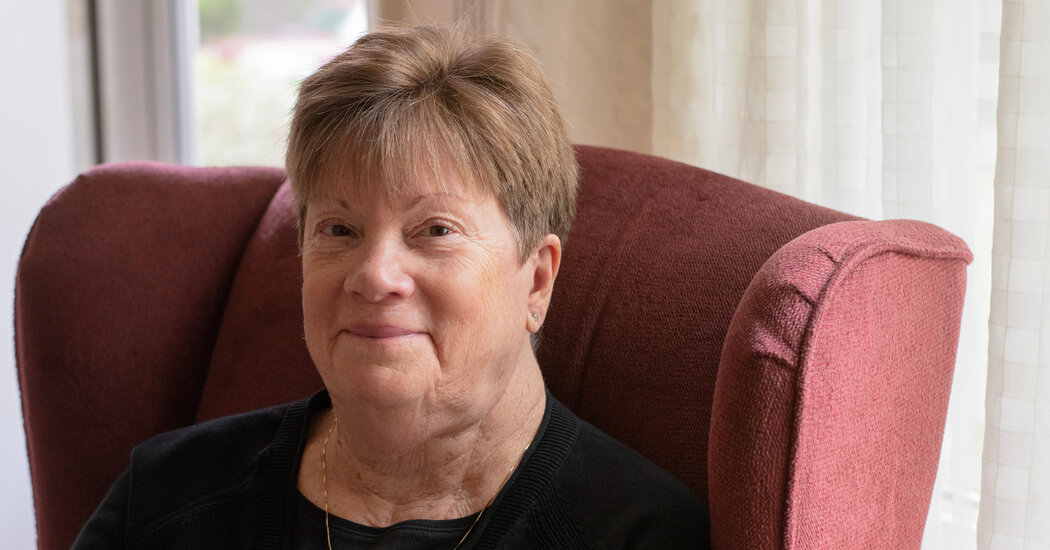The story, as often happens in science, sounded so appealing. Cells have a molecular clock that determines how long they live. If you can just stop the clock, cells can live indefinitely. And the same should go for people, who are, after all, made from cells. Stop the cell clocks and you can remain youthful.
The clocks come in the form of caps on the end of chromosomes — the long twisted strings of DNA carrying the cells’ genes. The caps on chromosomes, called telomeres, are chains of short, repeated segments of DNA. Every time a cell divides, its telomeres get a little shorter, until finally they get so short that the cell dies.
“Short telomeres were thought to be bad — people with premature aging syndromes had short telomeres — so, by analogy, long telomeres were thought to be good,” said Dr. Mary Armanios, professor of oncology at Johns Hopkins University School of Medicine and director of the Telomere Center at the medical school’s Sidney Kimmel Comprehensive Cancer Center. “And the longer the better.”
But, of course, nothing in biology is so simple. And a paper published Thursday in the New England Journal of Medicine, with results of a study that Dr. Armanios led, shows that the telomere story is no exception. While short telomeres do lead to health problems, long telomeres lead to health problems of their own. Far from extending life, long telomeres appear to cause cancer and a blood disorder known as CHIP, a condition that increases the risk of blood cancers and heart disease.
Dr. Elizabeth Blackburn, an emerita professor at the University of California, San Francisco, who shared a Nobel Prize for the discovery of an enzyme involved in making telomeres and who was not involved in the study, said it was a “beautiful paper” that went beyond correlations to show a direct link between long telomeres and disease. She added that the research “enlightens this whole trade-off.”
For Dr. Armanios, it is the culmination of work she began 20 years ago.
When scientists started studying telomeres, they observed that young people had longer ones than older people. When cells are grown in the lab, their telomeres act as sort of a ticking clock, determining how long they have to live.
Soon, telomeres were hailed as a secret to aging — companies advertised that they could tell your biological age by measuring the length of your telomeres. Others said that you could extend your life by preserving your telomeres with supplements.
But Dr. Armanios and other researchers had noticed that telomere lengths seemed constrained to a narrow range, indicating there is a price to pay for very long or very short telomeres.
Population studies by several groups seemed to support that idea. They found correlations — not a cause and effect — with increased disease risks at either end of the normal telomere spectrum.
Those with shorter than average telomeres appeared to have an increased risk of immune system problems and a variety of degenerative diseases, as well as pulmonary fibrosis, a lung disease. Those with longer than average telomeres appeared to have a modestly increased risk of cancer.
There were, though, some puzzlements.
“Some organisms have crazy long telomeres, like mice,” said Dr. Benjamin Ebert, chairman of medical oncology at the Dana-Farber Cancer Institute. “And mice don’t live that long.”
Dr. Armanios, as a human geneticist, thought the way to get answers was to study humans. “There are things you just can’t infer from studying cells,” she said.
She suspected, she said, that “you just can’t elongate telomeres without a price,” and began looking for people with very long telomeres to ask what that price might be.
She decided to look for people with a common genetic mutation, POT1, that can result in long telomeres. It was known to increase cancer risk but most researchers thought it was for reasons other than lengthening telomeres.
She ended up with 17 people from five families. They ranged in age from 7 to 83 and had extraordinarily long telomeres.
They also had tumors, ranging from benign, like goiters and uterine fibroids, to malignant, like those from melanoma and blood cancers. During the two-year study, four patients died of a variety of cancers.
Harriet Brown, 73, of Frederick, Md., is one of the study participants with very long telomeres. She has had benign tumors called paragangliomas in her neck and throat, thyroid cancer and two melanomas. She also has CHIP, the blood disorder associated with heart disease and blood cancers.
She has frequent scans and exams but, she said, “there is really not much I can do at this point,” because there is no way to prevent more tumors from developing.
The effects of long telomeres on people like Ms. Brown make perfect sense, said Dr. Norman Sharpless, professor of cancer policy and innovation at the University of North Carolina School of Medicine and a former director of the National Cancer Institute.
“It’s not that long telomeres make cells grow,” he said. “It’s that they don’t have the brakes to make them stop growing.” And because the telomeres of people with POT1 mutations do not grow shorter with each cell division, the cells hang around, dividing regularly. The longer they are dividing in the body, the more time they have to accumulate random mutations, some of which prompt tumor growth.
That’s especially true in blood, where cells are constantly being produced. POT1 mutations in some of these blood cells can give them time to accumulate other mutations that give them a selective advantage in growth. Soon some of these mutated blood cells pretty much take over a person’s bone marrow. The result is CHIP.
That is a new view of CHIP. The thought had been that because people with CHIP were at increased risk for blood cancer, that CHIP itself was causing cancer.
Instead, Dr. Armanios said, it’s that long telomeres are both creating CHIP and, independently, giving cells time to develop cancer-causing mutations.
“Aging biology is a lot more complicated than we’d hoped,” Dr. Sharpless said.
Or, as Dr. Blackburn observed: Long telomeres are not the secret to eternal youth.
“There is no free lunch,” she said.