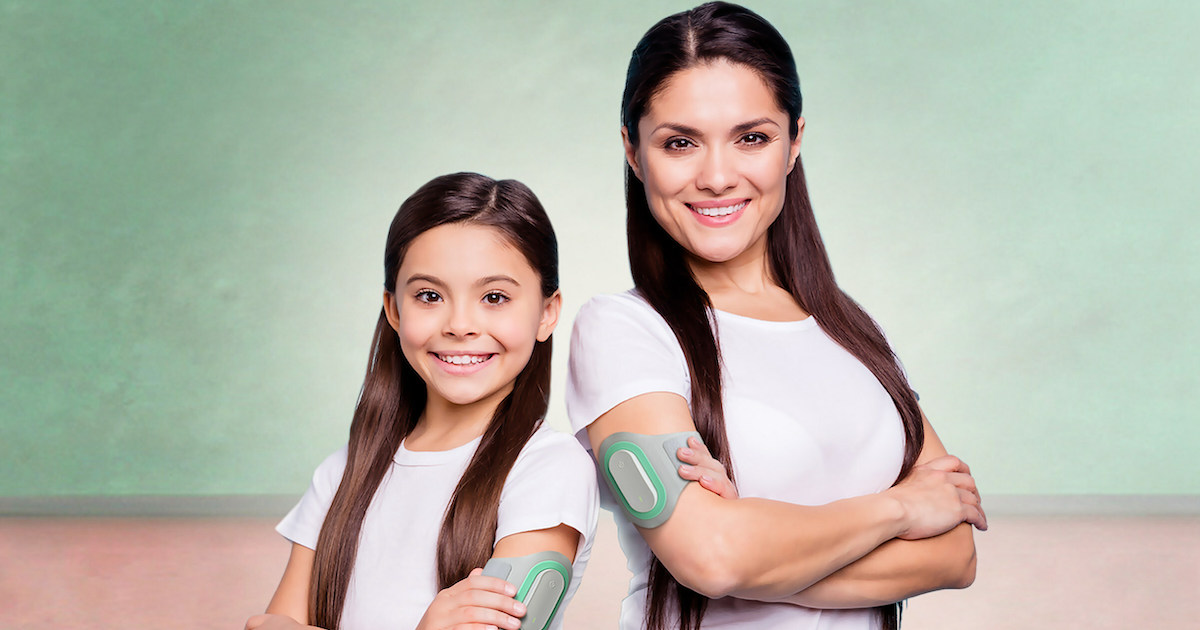Prescription digital therapeutic developer Theranica announced it has received FDA 510(k) clearance to use its Nerivio device for migraine prevention in patients 12 and older.
The wearable is worn around the upper arm and controlled with a smartphone. It delivers electrical pulses to stimulate small peripheral nerves, which aims to trigger a conditioned pain modulation response to alleviate symptoms. The treatment lasts 45 minutes and can be used every other day for prevention or at the onset of a migraine for acute treatment.
The device first received FDA De Novo clearance in 2019 to alleviate migraines once they’ve begun, and Theranica has since received additional clearances to remove a limitation for patients with chronic migraine and allow for adolescent use.
The company conducted a clinical trial with 248 participants that found patients using the Nerivio device every other day experienced fewer days with migraines over the eight-week study period compared with participants using a placebo wearable.
“Nerivio is paving a bold path forward in migraine treatment and prevention for adolescents and adults. We are hopeful this expanded dual-use indication will have a tremendous impact on mitigating the burden of migraine symptoms and improving patient quality of life,” Alon Ironi, Theranica’s CEO and cofounder, said in a statement.
THE LARGER TREND
Founded in 2016, Theranica announced it had received $45 million in Series C funding in August. The company, which has offices in Israel in New Jersey, said it plans to use the capital to expand its presence in the U.S. It previously raised $35 million in a Series B round in 2019.
About 12% of Americans have migraines, but they’re more common in women, people with a family history and those with other medical conditions like depression, anxiety, bipolar disorder, sleep disorders and epilepsy.
Other companies focused on migraine care include Germany-based Perfood, Thirty Madison with its Cove brand and Healint’s Migraine Buddy app.