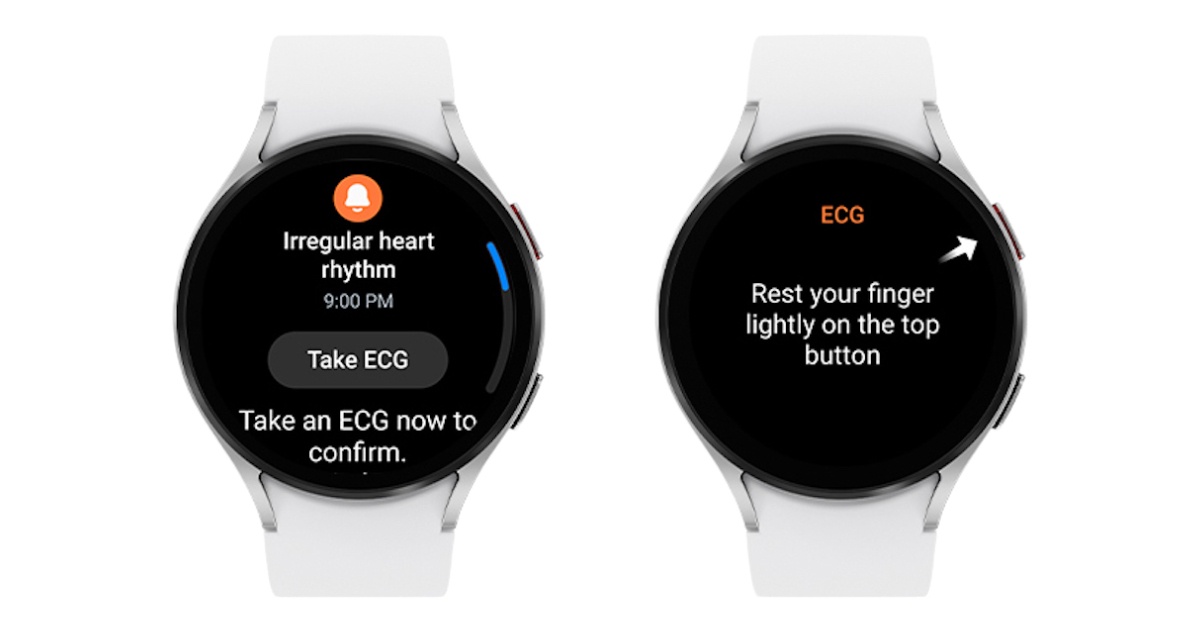
Tech giant Samsung Electronics announced it received FDA clearance for its Health Monitor app’s Irregular Heart Rhythm Notification (IHRN) feature.
IHRN works with the app’s electrocardiogram function to monitor heart rhythms suggestive of atrial fibrillation, an irregular heart rhythm. If AFib is detected, the Galaxy Watch prompts the user to take an ECG with the Watch.
The feature will be available as part of the recently announced One UI 5 Watch, slated to be released later this year.
“This is yet another example of how Samsung prioritizes proactive safety solutions and enables users to receive a more holistic understanding of their cardiovascular and overall health,” Hon Pak, vice president and head of the digital health team, MX business at Samsung Electronics, said in a statement.
THE LARGER TREND
Samsung’s One UI 5 Watch provides three new sleep-related features as well as a personalized heart rate zone, which analyzes a user’s physical capabilities and sets optimal workout intensity levels. Updated SOS and fall detection features were also announced.
Google-owned Fitbit also offers AFib features. Last year, it received FDA clearance for a photoplethysmography AFib detection algorithm, designed to run passively on Fitbit wearables and assess heart rhythm when a person is sleeping or not moving.
In September, Apple revealed its Watch wearables’ latest iterations, which include temperature-sensing capabilities to track ovulation and car crash detection. Months prior, it previewed new health features for watchOS 9 and iOS16, including atrial fibrillation history, medication management and more tools for sleep monitoring.